Background
ASCEMBL trial has demonstrated superior efficacy of Asciminib (ASC) over Bosutinib in terms of molecular response, and event-free survival. Furthermore, ASC could reduce discontinuation rates due to lower occurrences of adverse events (7% vs 25%). However, it remains to be established how ASC would compare to Ponatinib (PON), which is frequently used in chronic myeloid leukemia (CML) practice for patients (pts) failing multiple lines of tyrosine kinase inhibitor (TKI) therapy. No prospective study has been performed or is ongoing comparing ASC vs PON, but alternatively, observational data can provide valuable insight on this topic. The propensity score is the probability of therapy assignment conditional on observed baseline characteristics. We performed a propensity score matching (PSM) analysis to balance the variables affecting the treatment choice among ASC vs PON therapy. The present study aimed to compare the therapeutic efficacy of ASC to PON in CML pts who failed prior TKIs concerning molecular response, failure-free survival (FFS), progression, and overall survival (OS).
Patients and methods
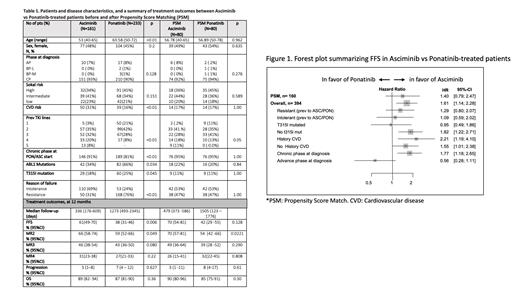
A total of 426 CML pts who had been treated with ASC or PON in 6 countries (Canada, Netherlands, Czech Republic, France, Argentine, and Italy) were included. In this preliminary report, data from 394 pts were analyzed. Primary endpoint was FFS at 1 year which was defined as the interval from TKI therapy start until treatment failure or death. Treatment outcomes were assessed for molecular response and long-term outcomes such as MR2 (BCR::ABL1 <1%IS or molecular response 2 log or deeper), MMR (BCR::ABL1 <0.1%IS or molecular response 3 log or deeper), MR4 (BCR::ABL1 <0.01%IS or molecular response 4 log or deeper), FFS, Progression, and OS. These parameters were evaluated in the overall population (n=394), resistant (n=254), or intolerant subgroups (n=126), T315I mutated (n=89) or non-T315I mutated subgroups (n=304), those having a past history of cardiovascular disease (CVD) (n=89) or not (n=304). For propensity score calculation, the following pre-treatment variables were selected using a binary logistic regression model: age, history of cardiovascular disease (CVD), disease phase, T315I mutation, and reason for failure to prior TKI line. 160 pts (i.e. 80 case-control pairs) were finally extracted through the PSM process within 0.2 of caliper difference.
Results
Significant differences were observed in various factors between ASC vs PON group, such as age (p<0.01), past history of CVD (p<0.01), previous TKI lines (p<0.01), presence of mutations (p=0.034), and reason for failure to prior TKI line (p<0.01). These differences ceased to exist when PSM was applied (Table 1). In the overall population, at 12 months, FFS was 46.7%, 95%CI [40.0-52.0%], MR2 62.6% [57.1-67.6%], MMR 44.7% [39.2-50.1%], MR4 28.6% [23.9-33.6%]), and OS 88.1% [84.1-91.1%].
In the overall population, PON group showed a lower FFS than ASC group (HR of PON 1.61, 95%CI [1.14-2.28], p=0.006), but, this difference was no significant when limited to the PSM selected pts. Figure 1 illustrates FFS in Asciminib vs Ponatinib-treated patients. In a subgroup of pts without T315I mutation, those on ASC showed higher FFS (HR of PON 1.82, [1.21-2.71], p=0.003), higher MR2 rate (HR of PON 0.69, [0.511-0.94], p=0.020), and higher MMR rate (HR of PON 0.677, [0.47-0.97], p=0.035). Additionally, in a subgroup of pts with a history of CVD those on ASC showed higher FFS (HR of PON 2.21, [1.19-4.10], p=0.011). Also, in pts in the chronic phase, ASC group showed higher FFS (HR of PON 1.77, [1.18-2.65], p=0.005), MR2 rate (HR of PON 0.73, [0.55-0.97], p=0.032), and MMR rate (HR of PON 0.71, [0.52-0.99], p=0.047). In the PSM balanced population, only MR2 rate was found to be significantly higher in favor of ASC (HR of PON 0.6, [0.39-0.93], p=0.022), while no significant differences were observed in all the other endpoints of interest.
Conclusion
From our current preliminary results, it can be established that ASC has at least equal efficacy to PON regarding FFS, MMR, MR4, progression, and OS which was confirmed in a PSM cohort. These findings contribute valuable insights to the understanding of ASC and PON treatments in pts with specific disease characteristics and may have implications for personalized therapy decisions in the absence of clinical trials comparing these drugs. Our aim is to expand our PSM cohort.
Disclosures
Žáčková:Angelini Pharma: Consultancy, Honoraria, Other: travel grant, Speakers Bureau; Pfizer: Honoraria, Other: travel grant, Speakers Bureau; Astra Zeneca: Other: travel grant; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel grant, Speakers Bureau. Nicolini:Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; INCYTE BIOSCIENCES: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; SUN pharma: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees. Castagnetti:Bristol Myers Squibb: Honoraria; Incyte: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding. Pavlovsky:Bristol Myers Squibb: Speakers Bureau; Pfizer: Speakers Bureau; Novartis: Speakers Bureau. Breccia:Novartis: Honoraria; Incyte: Honoraria; Pfizer: Honoraria; BMS: Honoraria; AOP: Honoraria; AbbVie: Honoraria. Rea:Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; INCYTE BIOSCIENCES: Consultancy, Honoraria, Speakers Bureau; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Westerweel:Pfizer: Honoraria; Novartis: Honoraria; Bristol Myers Squibb - Celgene: Honoraria; Incyte: Honoraria. Kim:BMS: Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Paladin: Consultancy, Research Funding; Novartis: Consultancy, Honoraria, Research Funding.